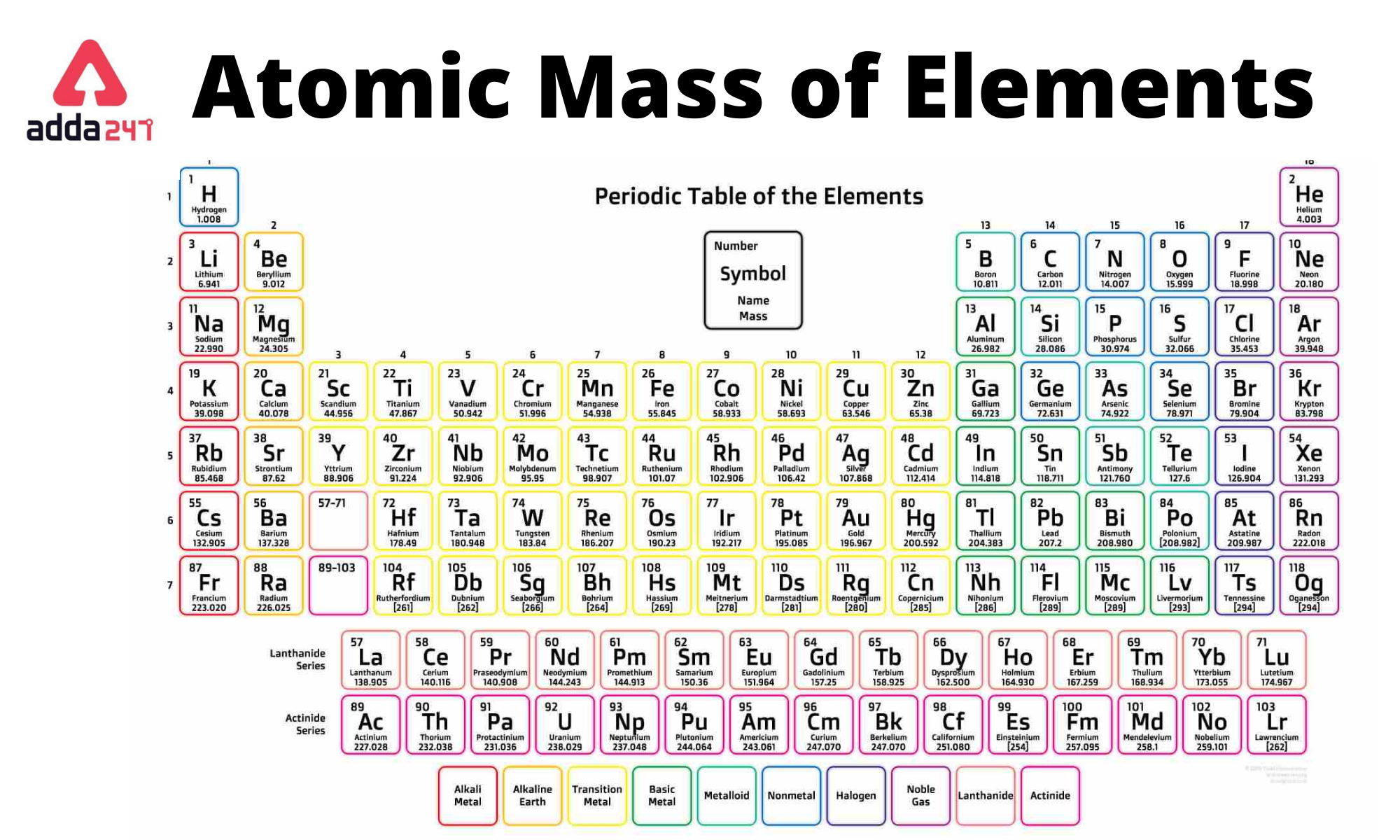
What is Atomic Mass? - List of Elements Sorted by Atomic Mass of Iron, Sulphur, Potassium, Chlorine Etc

Atomic Number & Mass Number | How to Find the Atomic Mass Number - Video & Lesson Transcript | Study.com
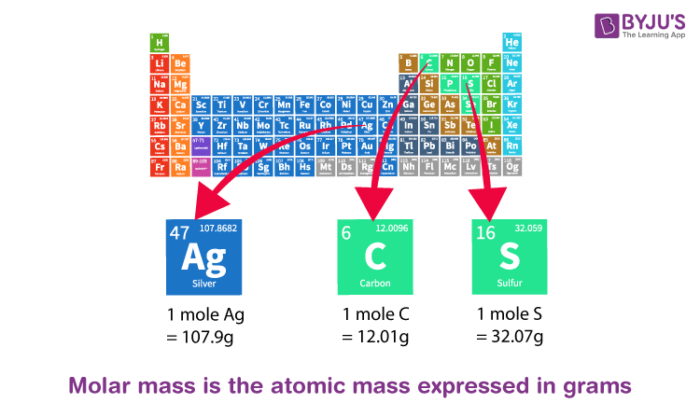
What's the math behind the similar numerical values of molar mass and relative molecular/atomic mass? - Quora

SOLVED: Atomic Number of an element is equal to:-(a) Number of Protons (b) Number of electrons(c) Number of neutrons (d) Both a) and b)

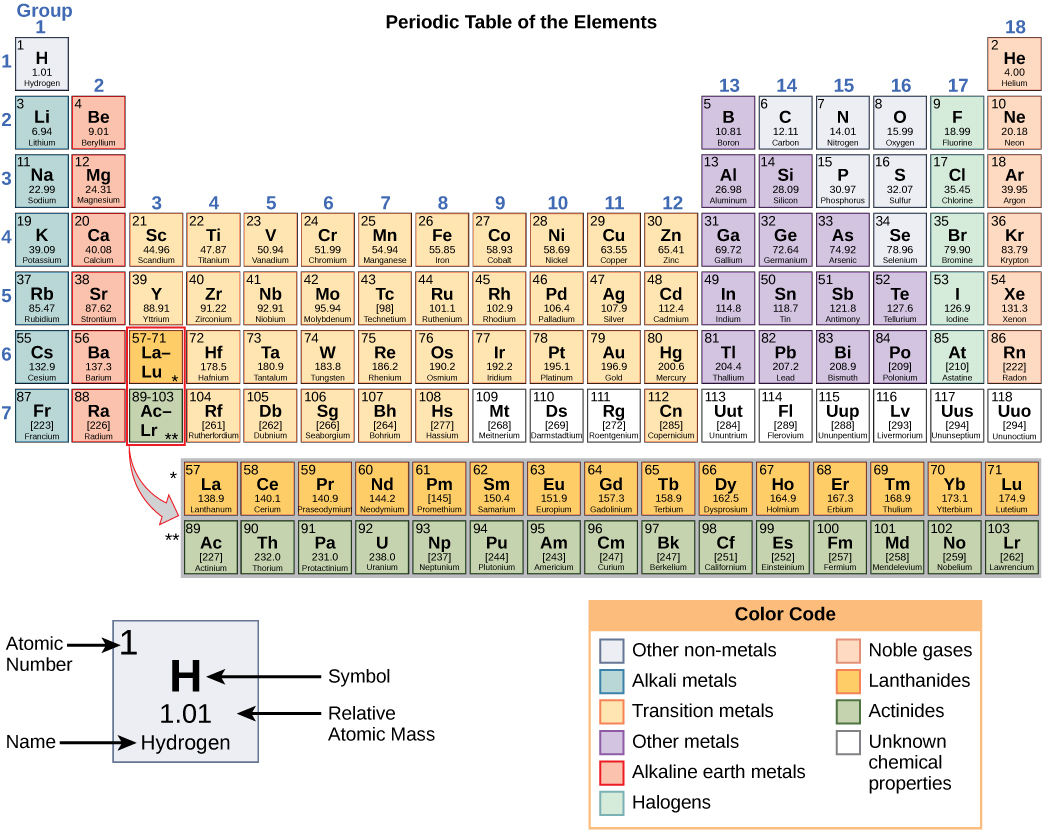
1 Atomic Mass The atomic mass of an element is listed below the symbol of each element on the periodic table. gives the mass of an “average” atom of each. - ppt download


:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)


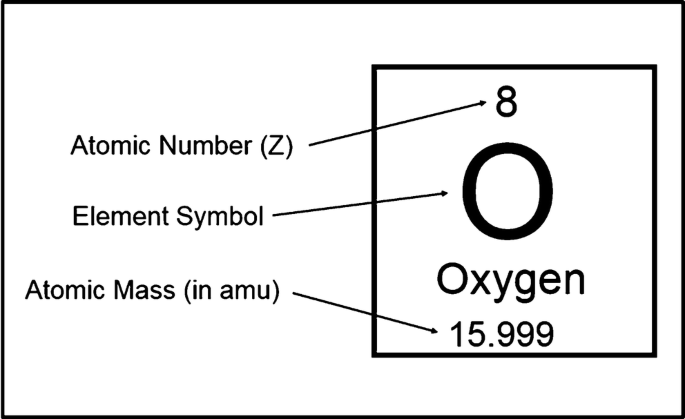
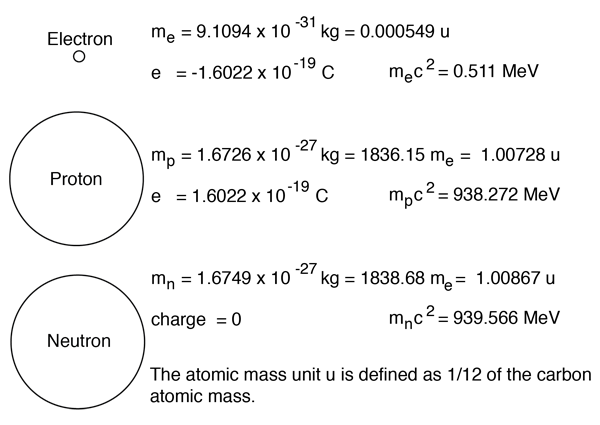



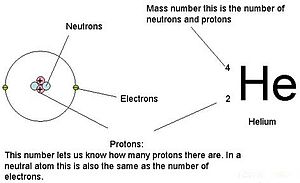

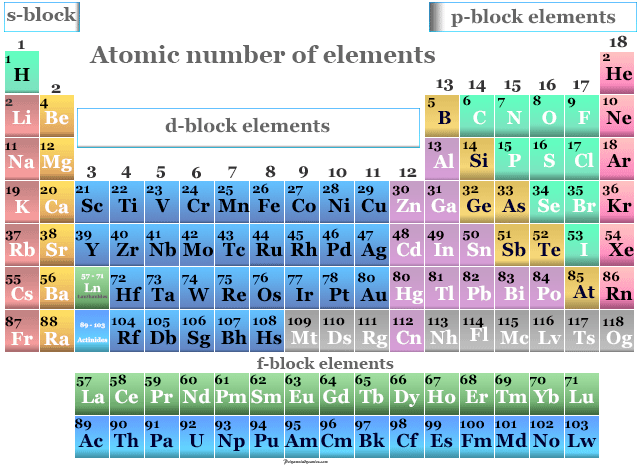
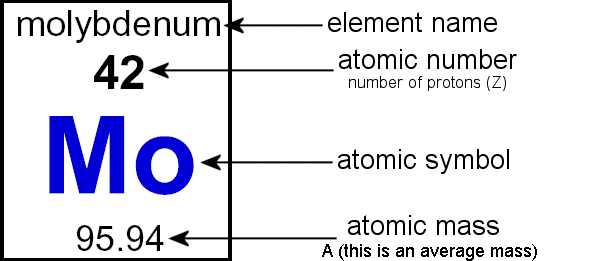
:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)